|
|
|
Yuri D. Ivanov, Vadim Y. Tatur, Ivan D. Shumov, Andrey F. Kozlov, Anastasia A. Valueva, Irina A. Ivanova, Maria O. Ershova, Nina D. Ivanova, Igor N. Stepanov, Andrei A. Lukyanitsa, Vadim S. Ziborov
1 Institute of Biomedical Chemistry, Moscow, Russia
2 Joint Institute for High Temperatures of the Russian Academy of Sciences, Moscow, Russia
3 Foundation of Perspective Technologies and Novations, Moscow, Russia
4 Moscow State Academy of Veterinary Medicine and Biotechnology Named after Skryabin, Moscow, Russia
5 Faculty of Computational Mathematics and Cybernetics, Moscow State University, Moscow, Russia
Abstract
The present study is aimed at the revelation of subtle effects of steam flow through a conical coil heat exchanger on an enzyme, incubated near the heat exchanger, at the nanoscale. For this purpose, atomic force microscopy (AFM) has been employed. In our experiments, horseradish peroxidase (HRP) was used as a model enzyme. HRP is extensively employed as a model in food science in order to determine the influence of electromagnetic fields on enzymes. Adsorption properties of HRP on mica have been studied by AFM at the level of individual enzyme macromolecules, while the enzymatic activity of HRP has been studied by spectrophotometry. The solution of HRP was incubated either near the top or at the side of the conically wound aluminium pipe, through which steam flow passed. Our AFM data indicated an increase in the enzyme aggregation on mica after its incubation at either of the two points near the heat exchanger. At the same time, in the spectrophotometry experiments, a slight change in the shape of the curves, reflecting the HRP-catalyzed kinetics of ABTS oxidation by hydrogen peroxide, has also been observed after the incubation of the enzyme solution near the heat exchanger. These effects on the enzyme adsorption and kinetics can be explained by alterations in the enzyme hydration caused by the influence of the electromagnetic field, induced triboelectrically by the flow of steam through the heat exchanger. Our findings should thus be considered in the development of equipment involving conical heat exchangers, intended for either research or industrial use (including miniaturized bioreactors and biosensors). The increased aggregation of the HRP enzyme, observed after its incubation near the heat exchanger, should also be taken into account in analysis of possible adverse effects from steam-heated industrial equipment on the human body.
Keywords:
horseradish peroxidase; enzyme aggregation; atomic force microscopy; triboelectric effect; coiled heat exchanger; superheated steam
1. Introduction
The motion of various liquid [1,2,3,4,5,6,7,8], gaseous [9,10], and two-phase [11,12,13,14] media along solid surfaces is known to cause the so-called triboelectric effect, which consists in the generation of an electric charge. The triboelectric effect in liquid media is now actively studied, being utilized in triboelectric nanogenerators [3,4,5,12,13,15,16]. The electric charge, generated in such a way, accordingly induces electric/electromagnetic fields. In this regard, the occurrence of electromagnetic fields induced triboelectrically upon the motion of water [6,17] and non-aqueous liquids [7,8,18,19] through pipes—including coiled ones [6,7]—should be mentioned. Coiled pipes (or simply coils) find numerous applications in heat exchanging equipment [20,21,22]. These heat exchangers can be organized in the form of cylindrical [22] and conical [23,24,25] coils.
In industrial coil heaters, steam is often employed as a heat-transfer agent [26]. In this connection, one should emphasize the occurrence of significant electrostatic effects upon the motion of steam [27,28,29,30]. These effects can even cause emergency situations in industry [31]. Accordingly, further investigation of these effects is required in order to develop safety standards regulating the steam-carrying equipment operation.
Electromagnetic [32,33,34,35,36,37,38,39] and magnetic [40,41,42,43] fields are known to affect physicochemical properties of enzymes. With regard to triboelectrically induced fields, they were reported to influence adsorption properties [6,7,8] and enzymatic activity [7] of horseradish peroxidase (HRP), which is often used as a model in studying the effects of electromagnetic and magnetic fields on enzymes [6,7,8,32,33,34,36,37,38,39,40,41,42,43]. Enzyme systems play key roles in the regulation of metabolic processes in the body [44]. This is why it is quite important to study the possible influence of electromagnetic fields, induced in steam-carrying heat exchangers, on enzyme systems.
The study of peroxidases is of great interest because these enzymes are well-represented in plant and animal tissues [44] and play important functional roles in the body. In the human body, in particular, an important role of myeloperoxidase involved in atherogenesis should be mentioned [45]. HRP is a ~44 kDa heme-containing enzyme [46,47], which is widely employed as a model in food science [36,37] in order to determine the influence of electromagnetic fields on enzyme systems [36,37,38,39]. HRP finds numerous applications in biotechnology [48,49] and in miniaturized biosensor systems [50,51], and this is another reason why it is extensively studied.
In the present work, with the example of HRP, we investigated whether the motion of steam through a conical heat exchanger affects the properties of the enzyme. The solution of HRP was incubated either near the apex or at the side of the conically wound aluminium pipe, through which steam flow passed. In order to study the adsorption properties and aggregation state of HRP before and after the incubation of its solution near the heat exchanger, atomic force microscopy (AFM) was used, while the HRP enzymatic activity was studied by spectrophotometry.
Owing to its ultra-high (0.1 nm) height resolution, AFM represents a powerful tool, which is widely employed for single-molecule investigation of enzymes [52,53,54,55,56,57,58,59,60,61,62]. In this way, AFM was employed to investigate the immobilization of ferredoxin-NADP+ reductase [52] and HRP [53] onto silanized mica. AFM was widely employed to reveal the aggregation state of HRP [6,7,8,32,33,34] and CYP102A1 [54] enzymes, and to study complex formation in the CYP11A1 enzyme system [55]. Berge et al. revealed a dimerization of the EcoKI enzyme after its binding with a DNA containing two recognition sites for the enzyme—as opposed to the case with a DNA containing one recognition site, when only a monomeric enzyme was observed [56]. By high-speed AFM, Crampton et al. visualized the interaction of EcoP15I with DNA, revealing two distinct mechanisms of this interaction [57]. By AFM, van Noort et al. [58] observed association, dissociation, and movement of photolyase over DNA macromolecules. Furthermore, in a number of publications, Radmacher and colleagues reported the use of an AFM-based approach for the direct observation of enzyme activity, which manifested itself in the form of height fluctuations of enzymes upon their interaction with respective substrates [59,60]. Namely, 1 nm height fluctuations of lysozyme macromolecules were revealed in the presence of an oligosaccharide substrate; moreover, such fluctuations were not observed without the substrate, or in the presence of lysozyme inhibitor chitobiose [59]. Measuring such height fluctuations allows one to directly observe single catalytic events of the enzyme; this has also been demonstrated with the example of chitosanase from Streptomyces griseus [60]. In [61], with the example of urease enzyme, immunoglobulin G, and microtubules, differences in height fluctuations above different macromolecules were revealed. Moreover, the use of AFM for studying lateral drift rate of urease macromolecules on silanized glass substrates was demonstrated [61]. Ivanov et al. [62] revealed that the amplitude of height fluctuations of oligomeric CYP102A1 enzyme was higher than that of monomeric CYP102A1 in the first 100 s of the enzyme functioning. After 100 s, a drop in the height fluctuation amplitude was observed, and this drop was explained by possible self-degradation of the enzyme [62].
The above-mentioned studies clearly demonstrate the ability of AFM to reveal even subtle effects of external factors on enzyme macromolecules [34]. Such subtle effects are often indistinguishable by macroscopic methods and can only be revealed by nanotechnology-based methods such as AFM [6,32,33,34]. This is why this method has been employed herein. This study has been aimed at the investigation of the influence of steam flow in a conical coil heat exchanger on individual HRP macromolecules incubated in its vicinity. The adsorption of HRP on mica has been investigated by AFM at the level of individual enzyme macromolecules. In parallel, spectrophotometry measurements of the HRP enzymatic activity in solution have been performed. Figure 1 displays the general workflow of the experiments performed.
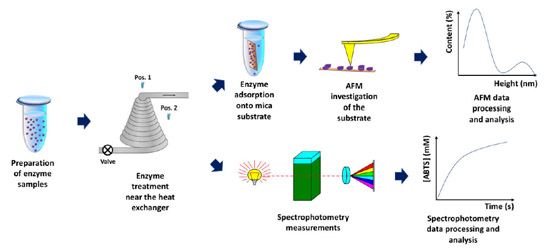
Figure 1. Schematic representation of general workflow of the experiments performed in order to investigate the influence of steam flow in conical heat exchanger on HRP enzyme.
By AFM, we demonstrated that the flow of superheated steam in the conical coil affects the adsorption properties of HRP macromolecules on mica. Namely, for the first time, an increased aggregation of the HRP enzyme on the mica substrate has been observed by AFM after its incubation either near the top or at the side of the conical heat exchanger. At the same time, such an incubation has been found to cause a change in the shape of the kinetic curve reflecting the HRP-catalyzed oxidation of its substrate 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS). The results obtained herein should be taken into account in the development of equipment involving conical heat exchangers, intended for either research or industrial use. Additionally, our data reported can also contribute to further analysis of possible adverse effects from steam-heated industrial equipment on human body.
Полный текст доступен в формате PDF (600Кб)
Ivanov, Y.D.; Shumov, I.D.; Tatur, V.Y.; Valueva, A.A.; Kozlov, A.F.; Ivanova, I.A.; Ershova, M.O.; Ivanova, N.D.; Stepanov, I.N.; Lukyanitsa, A.A.; Ziborov, V.S. AFM Investigation of the Influence of Steam Flow through a Conical Coil Heat Exchanger on Enzyme Properties. Micromachines 2022, 13, 2041. https://doi.org/10.3390/mi13122041
Yuri D. Ivanov, Vadim Y. Tatur, Ivan D. Shumov, Andrey F. Kozlov, Anastasia A. Valueva, Irina A. Ivanova, Maria O. Ershova, Nina D. Ivanova, Igor N. Stepanov, Andrei A. Lukyanitsa, Vadim S. Ziborov, AFM Investigation of the Influence of Steam Flow through a Conical Coil Heat Exchanger on Enzyme Properties // «Академия Тринитаризма», М.,
|
|