|
|
|
Vadim S. Ziborov, Tatyana O. Pleshakova, Ivan D. Shumov, Andrey F. Kozlov, Irina A. Ivanova, Anastasia A. Valueva, Vadim Yu. Tatur, Andrey N. Negodailov, Andrei A. Lukyanitsa, Yuri D. Ivanov
1 Institute of Biomedical Chemistry
2 Foundation of Perspective Technologies and Novations
Abstract
Atomic force microscopy is employed to study the influence of the motion of a glycerol solution through a coiled (spiral-wound) polymeric communication pipe on the aggregation state of a protein, with the example of a horseradish peroxidase (HRP) enzyme. The measuring cell with the buffered solution of the protein was placed within the experimental setup over the pipe coil, through which glycerol was pumped. It is demonstrated that, in such a system, the flow of a non-aqueous liquid (glycerol) leads to a change in the physicochemical properties of a protein, whose solution was incubated in the measuring cell placed over the coil. Namely, changes in both the adsorbability onto mica and the aggregation state of the model HRP protein were observed. As glycerol-containing liquids are commonly used in biosensor operations, the results reported herein can be useful to the development of biosensor systems, in which polymeric communications are employed in sample delivery and thermal stabilization systems. The data obtained herein can also be of use for the development of specified hydrodynamic models.
Keywords: liquid flow; atomic force microscopy sensor; protein aggregation; glycerol; horseradish peroxidase
1. Introduction
In biosensors and bioreactors, flow-based systems are commonly employed for the delivery of reagents [1,2], as well as for mass [3] and heat exchange [4]. For these purposes, aqueous or nonaqueous media can be employed, depending on the process’s design. In connection with this, in a number of papers, an occurrence of a triboelectric generation of charge upon the motion of liquid media along solid surfaces was demonstrated [5–12]. Such a triboelectric effect is known to occur upon the motion of both water and aqueous solutions [5–12], as well as of various non-aqueous liquids [13,14], including glycerol [14].
A triboelectric effect consists of a generation of electric charges in a material during friction. This generation represents contact electricity (electrization), where one material becomes electrically charged after its frictional contact with another material. Upon the frictional contact, a charge redistribution occurs. The mechanism of the appearance of triboelectric charges was explained by Helmholtz [15]. Namely, it is supposed that a charge transfer from one object to another object occurs upon their contact. This way, an electric field in an interface appears. This field is called a double layer, and the voltage within this layer is called contact voltage. Friction upon the electrization favors an increase in the area of the double layers. A triboelectric effect can occur upon friction between the following solid and/or liquid media: dielectric/dielectric; semiconductor/semiconductor; metal/metal (of different densities); metal/dielectric; liquid dielectric/liquid dielectric; liquid dielectric/metal; and liquid dielectric/solid dielectric. An example illustrating the latter case is the occurrence of the triboelectric effect upon the motion of water through a polymeric communication [5,16]; the motion of glycerol along a dielectric surface is also the same case [14].
As mentioned above, the motion of water along polymeric surfaces leads to a triboelectric generation of charge. The latter has been shown to influence the properties of biological macromolecules. Namely, the motion of water through biosensor communication pipes [16] and the flow of water through injectors into the biosensor measuring cell [17] were reported to influence the physicochemical properties of proteins. So, it was demonstrated that an electric charge is generated upon the injection of a protein solution through a polypropylene tip [17]. Such a generation of charge leads to an increase in the specific charge per protein molecule while decreasing its concentration, which correlates with a decrease in the protein detection limit, achievable with the biosensor system, down to 10-17 M [17]. Moreover, in our recent study, the flow of water through coiled communication pipes, located near the measuring cell containing a solution of horseradish peroxidase (HRP) protein, was shown to have an effect on the protein’s aggregation state [16]. Such a phenomenon was ascribed to the influence of an electromagnetic field induced upon the motion of water through the coiled communication pipe, owing to the triboelectric generation of charge. Such a generation of charge upon the flow of water through injectors was discussed in several studies [5,9,18].
Similar to water, glycerol represents an important substance widely used in biochemical studies of biological objects. As regards glycerol, its importance to biochemical research is determined by two factors. The first one is that glycerol is widely employed in studies of solutions of biological objects. For instance, glycerol was used to optimize the viscosity of solutions and the diffusivity of reagents in biosensors [19], as an emollient [20], and as a surfactant for the treatment of a sensor chip surface [21,22]. On the other hand, as was noted above, glycerol and glycerol-based solutions are commonly used in biosensor experiments involving proteins [2,19,22]. In addition, glycerolcontaining solutions are used in studies of enzymes; namely, enzymes are dissolved in glycerolcontaining solutions to preserve their native structure [23–26]. In the course of operations with such biological objects, the glycerol-containing solutions are aspirated and injected into a measuring cell using injectors. With respect to bioreactors and biosensors, it has to be noted that injector nozzles, communication pipes, and thermal stabilization tubing pipes in flow-based systems are often fabricated not only from metal, but also from various polymeric materials [1,2]. In this way, polydimethyl siloxane [27–30], polytetrafluoroethylene (Teflon [31]), polymethyl metacrylate [32], and nylon [33] represent typical polymers used for the fabrication of biosensor fluidic sections. Upon the motion of glycerol-containing solutions through polymeric fluidic lines, a charge is expected to be generated at the expense of the above-mentioned triboelectric effect, similar to the case with aqueous solutions.
The second factor is that glycerol-containing liquids can be employed as heat-transfer agents for thermal stabilization of biosensor measurement systems [34]. This is particularly important in the instrumentation of systems operating in extreme field conditions in a wide range of temperatures (from negative to positive ones), depending only on the glycerol content. This is possible due to the unique properties of glycerol, since the freezing point of such a heat-transfer fluid can be varied in a very broad range, from −43.5 °C (for 66.7% w/w glycerol solution [35]) to 17 °C (100% glycerol) [36]. As was mentioned above, the flowing of glycerol-containing solutions through the pipes of thermal stabilization systems is expected to cause a triboelectric effect, i.e., a generation of an electric charge. The latter, in its turn, should induce an electric field that can extend well beyond these communications, influencing protein solutions in biosensor systems or bioreactors.
The present study is aimed at determining the influence of the flow of glycerol through a coiled polymeric pipe on the physicochemical properties of the HRP solution in the measuring cell, which is located in the vicinity of the coiled pipe.
The HRP enzyme was employed as a model object, since this protein was comprehensively characterized in the literature [37–47]. The molecular weight of HRP, which represents a hemecontaining enzyme glycoprotein [37,38], is known to be 40 to 44 kDa [38,39]. The HRP molecule contains 18–27% carbohydrate chains, stabilizing its structure [38,40,41]. It is known that many enzymes, including peroxidase [42], form aggregates. Similar to our previous studies [16,48], atomic force microscopy (AFM) has been employed for the visualization of the effect of an electromagnetic field on proteins with the example of HRP macromolecules, which were adsorbed from the analyzed solution onto the atomically smooth surface of a mica substrate. AFM allows one to register a signal from even single-surface-adsorbed macromolecules, and this extremely high sensitivity has allowed us to detect minor changes in the properties of HRP macromolecules. In the present study, using AFM, it was found that the flow of glycerol (similar to water flow [16]) through the flow-based system influences the physicochemical properties of HRP macromolecules; namely, it induces changes in the HRP aggregation state and in its adsorbability onto the mica surface. The data obtained herein can be used in the development of biosensor systems intended for the diagnosis of socially relevant diseases (such as oncological and infectious ones). Our results can also be useful for the development of biosensors operating in a broad temperature range for the registration of changes in physiochemical properties of target proteins associated with these diseases. The effects observed in our experiments can also be taken into account for the development of models of fluid dynamics.
2. Materials and Methods
Reagents: Peroxidase from horseradish (HRP-C; Cat.# P6782) was obtained from Sigma (St. Louis, MO, USA). In the experiments, we used a 0.1 μM solution of HRP in a 2 mM PBSD buffer (Pierce, USA), prepared by sequential ten-fold dilution of a 10-5 М stock solution. The latter was prepared by dissolving 0.15 mg of an initial HRP preparation in 0.375 mL of the same buffer. At each dilution step, the protein solution was incubated in a shaker at 23 °С and 600 rpm for 30 min. In each experiment, 1 mL of 0.1 μM HRP solution was used.
Ultrapure water (with a resistivity of 18.2MΩ×cm), used throughout the study, was obtained with a Simplicity UV system (Millipore, Molsheim, France).
Glycerol (used as a working liquid to be pumped through the flow section of the experimental setup) was purchased from Glaconchemic GmbH (Germany).
2.1. Experimental Setup
The experimental setup is schematically shown in Figure 1. The setup included a model of a flow section of a biosensor with a coiled communication pipe and a measuring cell.
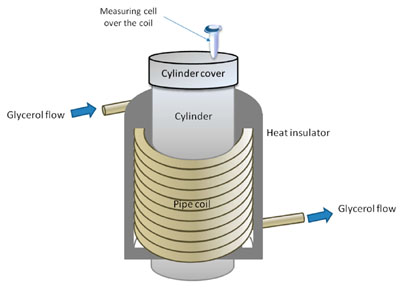
Figure 1. Experimental setup for studying the influence of glycerol flow through a coiled communication pipe on the physicochemical properties of horseradish peroxidase (HRP). The measuring cell was placed over the coil.
A KAN-therm PE-RT/AL/PE-RT pipe (KAN-therm, Poland) fabricated from armed polyethylene was used as a communication pipe. This pipe was fabricated from a more durable material than silicone (used in our previous study [16]) and hence could be successfully used in experiments with warm glycerol, as glycerol is a much more viscous liquid. The pipe coil’s dimensions were as follows: a coil diameter of 25 cm, a coil height of 40 cm, and an inner diameter of the pipe of 1.5 cm. To provide sufficient intensity of the glycerol flow through the pipe, the glycerol in the flow-based system was warmed to 65 °C to provide better flowability [49]. At the same time, the coiled pipe was covered with a thermal shield (a layer of metallized, foamed polypropylene) to avoid undesired heating of the measuring cell with the test protein solution, whose temperature remained unchanged and was maintained at a level of 23 ° С. To supply glycerol into the flow-based system, a BE-G 20 HP0.8 gear pump was employed. The volumetric flow rate of glycerol was 8.7 L/min.
In control experiments, the cell with the HRP solution was placed far from the experimental setup (at a distance of 10 m) for 40 min (analogously to [16]). After that, the measurements were performed analogously to the working experiments.
Полный текст доступен в формате PDF (899Кб)
Ziborov, V.S.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Ivanova, I.A.; Valueva, A.A.; Tatur, V.Y.; Negodailov, A.N.; Lukyanitsa, A.А.; Ivanov, Y.D. Investigation of the Influence of Liquid Motion in a Flow-Based System on an Enzyme Aggregation State with an Atomic Force Microscopy Sensor: The Effect of Glycerol Flow. Appl. Sci. 2020, 10 (14), 4825; https://doi.org/10.3390/app10144825
Vadim S. Ziborov, Tatyana O. Pleshakova, Ivan D. Shumov, Andrey F. Kozlov, Irina A. Ivanova, Anastasia A. Valueva, Vadim Yu. Tatur, Andrey N. Negodailov, Andrei A. Lukyanitsa, Yuri D. Ivanov, Investigation of the Influence of Liquid Motion in a Flow-Based System on an Enzyme Aggregation State with an Atomic Force Microscopy Sensor: The Effect of Glycerol Flow // «Академия Тринитаризма», М.,
|
|