|
|
|
Yuri D. Ivanov, Tatyana O. Pleshakova, Ivan D. Shumov, Andrey F. Kozlov, Tatyana S. Romanova, Anastasia A. Valueva, Vadim Yu. Tatur, Igor N. Stepanov, Vadim S. Ziborov
1 Institute of Biomedical Chemistry
2 Foundation of Perspective Technologies and Novations
Abstract: The influence of liquid motion in flow-based systems on the aggregation state of an enzyme and on its enzymatic activity was studied, with horseradish peroxidase (HRP) as an example. Our experiments were carried out in a setup modeling the flow section of the biosensor communication with a measuring cell containing a protein solution. Studies were conducted for a biosensor measuring cell located along the axis of a spiral-moving liquid flow. The aggregation state of the protein was determined with an atomic force microscopy-based sensor (AFM sensor). It has been demonstrated that upon flowing of water through silicone biosensor communications, an increased aggregation of HRP protein was observed, but, at the same time, its enzymatic activity did not change. Our results obtained herein are useful in the development of models describing the influence of liquid flow in biosensor communications on the properties of enzymes and other proteins. This is particularly important for the development of serologic protein biosensors, which are beginning to be used for the early diagnosis of oncological diseases (such as brain cancer, prostate cancer, breast cancer etc.). The results obtained herein should also be taken into account when considering possible changes in hemodynamics due to increased protein aggregation.
Keywords: horseradish peroxidase; atomic force microscopy sensor; protein aggregation
1. Introduction
In biosensor systems, spiral-coiled communications with a flowing aqueous medium are often used for thermal stabilization. Such communications are employed both in miniaturized biosensor systems and in large bioreactors [1]. Upon the flowing of water through spiral-wound communications, triboelectric effects occur [2–6]; the occurrence of such effects leads to the generation of an electric current. In turn, the motion of electric charges (i.e., the electric current) is known to induce electromagnetic fields. Despite these fields being weak, they nevertheless can influence biological objects. As is known, weak electromagnetic radiation, whose power is in the range of ~10−4 W, has an influence on human body (though this influence is weak)—so-called non-thermal effects [7,8]. Studying these effects is of great importance, since weak radiation is beginning to be used in medicine for disease treatment. For instance, pilot research is being undertaken to study the therapeutic effect of electromagnetic radiation of non-thermal power in cancer [7]. At the same time, the influence of weak electromagnetic radiation on enzyme systems is still insufficiently studied. So, a number of studies have concerned the aggregation state of proteins in biosensor systems [9,10], but the influence of inductive electromagnetic fields was not considered. On the other hand, it was demonstrated that a triboelectric effect appears upon flowing of water in polymeric pipes, which leads to a generation of charge [2–6]; accordingly, an electromagnetic field is induced. In modern biosensors, polymeric materials are widely used in fluidic communications, in thermostabilization systems etc.
Recent experimental studies showed that the triboelectric effect is observed not only for pure (deionized or distilled) water, but also for tap water [11–13] and even for 0.6 M NaCl and seawater [11,12], whose resistivity is low.
At that, obviously, biosensors are fabricated in the form of compact devices, to save materials, working space etc. Since an electric field is induced upon the flowing of a liquid through communication components, the influence of this field can spread beyond these components. Accordingly, it is of interest to find out the relationship between the process of the flowing of a liquid sample and the properties of the sample located, for instance, in the measuring cell.
Thus, in our present study, typical conditions occurring in a biosensor or in a bioreactor have been modeled, and the influence of flowing of water through spiral communications of a biosensor on the properties of a protein, with an example of a well-studied protein—horseradish peroxidase (HRP) enzyme—has been investigated. HRP pertains to heme-containing enzymes [14]. These enzymes play an important role in various metabolic processes in human [15]. Moreover, HRP is often employed in biosensor analysis as an enzymatic tag [16,17]. These are the reasons why this protein has been chosen as a model object for our study. In our present research, HRP has been employed as an object of study, since this protein is a well characterized enzyme often used as a model in studies of a wide class of peroxidases. The study of peroxidases is of great interest due to the fact that these enzymes are well represented in plant and animal tissues [18] and play an important functional role in the organism. Peroxidase catalyzes the oxidation of a broad spectrum of organic and inorganic compounds by hydrogen peroxide [19]. In particular, one should point out an important role of myeloperoxidase, which participates in atherogenesis in humans [20]. The molecular weight of HRP heme-containing glycoprotein is 40 to 44 kDa [21,22]. It is known that many enzymes, including horseradish peroxidase [23], form aggregates. It was demonstrated that a change in the aggregation state of HRP is observed upon the influence of electromagnetic fields with 100 Hz to 100 kHz frequencies. So, aggregation of this protein was observed in alternating magnetic field with 40 kHz frequency at various intensities from 510 to 1230 kA/m [24]. Neither a constant magnetic field nor alternating electric field with the frequency within the range from 100 Hz to 100 kHz caused any change in the aggregation state of HRP; however, a significant aggregation was observed in the case of the combination of these fields [25].
In this way, the aggregation state of HRP biomolecules can be used as an indicator of the effect of electromagnetic fields on biological molecules. A change in the aggregation state of an enzyme due to an external physicochemical impact—such as electromagnetic, thermal, and chemical— characterizes a change in its spatial structure. The latter can lead to changes in its functional properties and, as a consequence, to a pathological state of the whole organism.
To study the effect of the electromagnetic field on HRP aggregation, an atomic force microscopy-based sensor (AFM sensor) has been employed. The use of the AFM-based approach for studying the effect of weak knotted electromagnetic fields on the aggregation state of HRP was demonstrated in our recent study [26]. In this way, one more reason why this protein has been chosen as a model object for our present study is the fact that the aggregation state of HRP is rather sensitive to the influence of weak electromagnetic fields. The importance of data obtained upon monitoring protein aggregation consists of the possibility of the registration of minor effects, as every (even insignificant) change in protein structure can affect the aggregation state of the macromolecules. At that, changes in the structure are pronounced as an integral effect, which is connected with both the interaction between proteins in their aggregates and the protein–surface interactions. To study protein aggregation, light scattering-based methods such as dynamic light scattering (DLS) and multi-angle light scattering (MALS) are commonly employed. It is to be noted that these methods are used for the determination of protein aggregation in solution. In our present study, we employed AFM to determine the possibility of protein aggregation on the surface, since in such a case it is possible to observe additional effects of interaction of the protein with the AFM substrate surface. Due to such effects, the results obtained by DLS can differ from those obtained by AFM. In addition, it should be noted that, to ensure the intensity of the scattered light is not lower than the DLS device sensitivity threshold, DLS experiments often require much higher protein concentrations than those used in AFM experiments [27]. At the same time, concentration effects can significantly influence the HRP aggregation state [23,28]. This is another reason why the results obtained by AFM and by DLS can differ. In the framework of the present study, our task consisted of the revelation of changes in the protein's properties under the influence of electromagnetic field induced by the water flow at the single-molecule level. Due to the above-listed factors, these changes can be indistinguishable while using DLS; these changes, however, manifest themselves upon using AFM, when the signal from individual macromolecules is detected [29,30]. AFM allows one to visualize individual enzyme molecules [29,30], which is important for single molecule enzymology. To monitor the aggregation state of HRP in solution before and after its exposure to the field, atomic force microscopy visualization of HRP macromolecules, adsorbed from this solution onto atomically smooth substrate surface, was performed. In parallel, the enzymatic activity of HRP was monitored by conventional spectrophotometry. In our research, these two methods were employed together, as it was assumed that weak electromagnetic field can influence the properties of HRP as discussed above. However, in cases when this change does not affect either the active site or chromophoic groups of the enzyme, it is difficult to reveal such changes by measuring the kinetic parameters of the enzyme reaction.
Herein, we modeled the measuring cell of a biosensor (or a bioreactor vessel) using a standard polypropylene tube filled with HRP solution, while the polymeric communication was modeled with a silicon pipe, spiral-wound onto a glass cylinder to form a coil, through which tap water was pumped (see Figure 1). Such a geometry is often used in thermostabilization of biosensors’ elements, including measuring cells, and in a number of other cases. The cell was placed over the spiral-wound pipe parallel to the coil axis (over the coil) (Figure 1). Our AFM data indicated that in the case when the cell, containing HRP solution, was placed over the coil, an increase in the HRP protein structures (that is, HRP aggregation) was observed. At that, according to the spectrophotometric data, enzymatic activity of HRP did not change.
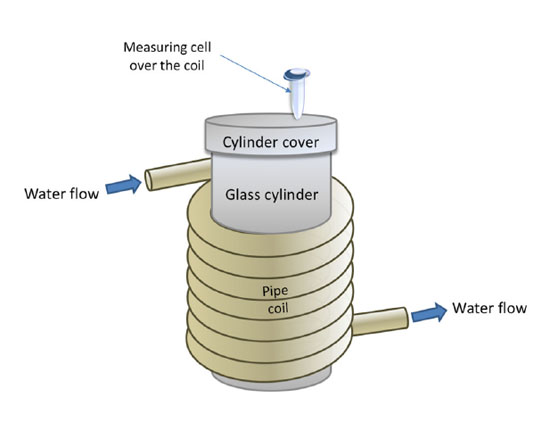
Figure 1. Schematic representation of the experimental setup employed for studying the effect of liquid flow on the properties of a protein. The measuring cell with the enzyme solution was placed over the coil; “water flow” marks indicate start and end section of the silicone pipe, which was spiral-wound onto a glass cylinder to form a coil; water was pumped through the coiled pipe.
Moreover, to study the inductive field (induced by the motion of water) along the communication axis, kinetic studies were conducted to measure the action of this field on a metal disk suspended on a cobweb thread along the axis of this communication. This system modeled highly sensitive torsion balance, commonly used in physics to study the induced fields by monitoring the torsion of a disc. It was obtained that the inductive field influences the position of the disc, making it spin. This motion was recorded on a video.
The results obtained herein should be taken into account in the analysis of the structure of proteins and their complexes studied using biosensors, in the development of serological diagnostics based on protein markers of diseases requiring early diagnosis, such as brain cancer, prostate cancer, etc. These data can also be of use in the study of hemodynamics in the human body.
Полный текст доступен в формате PDF (1032Кб)
Видео движения, ускоренного в 100 раз, доступно в формате MP4 (36840Кб)
Applied Sciences, 2020, 10(13), 4560;
https://doi.org/10.3390/app10134560
Yuri D. Ivanov, Tatyana O. Pleshakova, Ivan D. Shumov, Andrey F. Kozlov, Tatyana S. Romanova, Anastasia A. Valueva, Vadim Yu. Tatur, Igor N. Stepanov, Vadim S. Ziborov, Investigation of the Influence of Liquid Motion in a Flow-Based System on an Enzyme Aggregation State with an Atomic Force Microscopy Sensor: The Effect of Water Flow // «Академия Тринитаризма», М.,
|
|